Novel Biologics
Innovation That Moves Us
At Dr. Reddy’s Biologics, we are committed to furthering global healthcare with cutting-edge therapies that address unmet medical needs. Our journey into New Biological Entities (NBEs)/ Novel Biologics reflects our dedication to advancing science and improving patient outcomes.
Our Journey
Our Novel Biologics Products Shelf
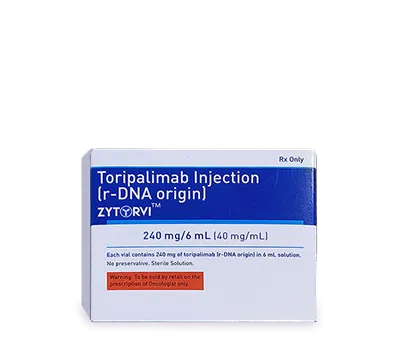
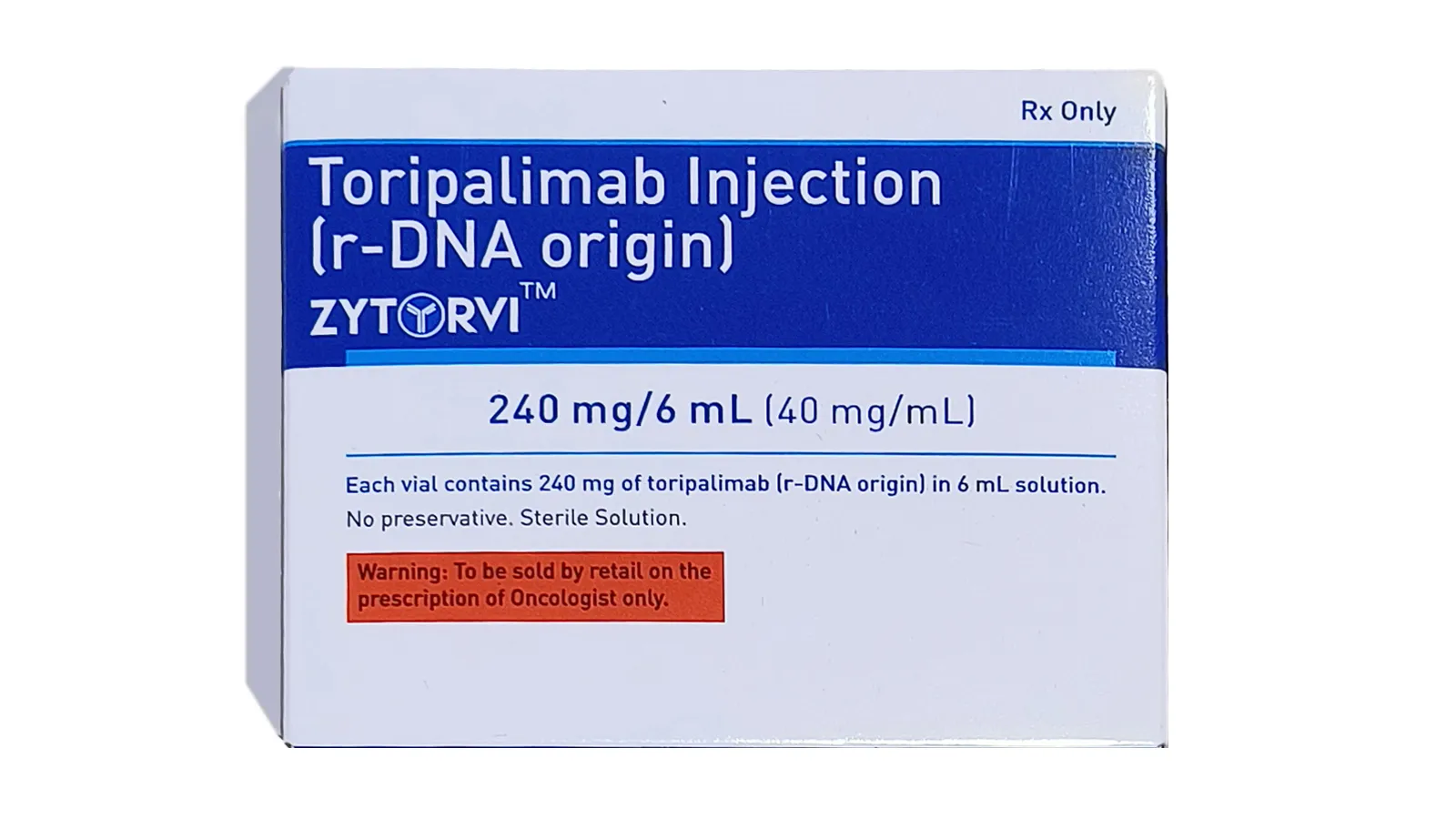
Zytorvi
In treatment of adults with recurrent or metastatic nasopharyngeal carcinoma
(RM-NPC), Zytorvi, our brand name for Toripalimab, is a path-breaking
solution. USFDA, EMA, MHRA approved.
Our Novel Pipeline
In 2023, Coya Therapeutics, Inc. and Dr. Reddy’s Laboratories entered into an exclusive collaboration for the development and commercialisation of COYA 302, an investigational combination therapy for the treatment of Amyotrophic Lateral Sclerosis (ALS).
COYA 302 is a biologic designed for subcutaneous administration, combining low-dose IL-2 and CTLA4-Ig (abatacept). With a dual mechanism of action, it aims to suppress chronic and sustained inflammation associated with certain neurodegenerative diseases while effectively modulating immune responses.
This ground-breaking therapeutic offers a promising solution for patients with complex and challenging conditions, addressing unmet medical needs through innovative science.
- **Co-developed
Product
Therapeutic Area
Type
Early Development
Pre Clinical
Phase1
Phase2
Phase3
Under Approval
Coya 302**
ALS
New Biological Entity
We Meet Global Standards
What Lies Ahead
At Dr. Reddy’s Biologics, our mission goes beyond delivering therapeutic solutions. We are shaping the future of Biologics with world-class R&D capabilities, state-of-the-art manufacturing facilities, and a robust global network. With breakthrough molecules like Toripalimab, COYA 302, and others in our pipeline, we aim to redefine healthcare and bring transformative therapies to patients. Guided by our vision of GOOD HEALTH CAN’T WAIT, we are dedicated to creating a lasting impact on the healthcare landscape.
What guides us:

Patient-Centric Approach: Addressing complex diseases with unmet medical needs to improve patient outcomes.

Cutting-Edge Science: Harnessing advanced technology platforms and deep R&D expertise to drive innovation.

Global Impact: Committed to making high-quality biologics accessible to patients worldwide.
- News
- 17-12-2023
Dr. Reddy's, Coya Therapeutics Ink Licensing Agreement For ALS Treatment Drug
- News
- 06-12-2023
Coya taps familiar friend Dr. Reddy's to commercialize ALS med
- News
- 29-07-2024
Dr. Reddy’s biosimilar gets recommendation from European Medicines Agency
- News
- 29-07-2024
Dr Reddy’s gets positive opinion from EMA for its rituximab biosimilar

Chandana Priya
Quality

Saikiran Kodoori
Product Dev.

Harini M
Product Dev.

Arpita Sarkar
Manufacturing

Anjani Srinivas
Engineering
Bricks of Biologics, appreciates the contribution and celebrates our people who are the building blocks of Dr. Reddy's Biologics.
Bricks of biologics in general refers to bio-bricks as genetic building blocks which are DNA sequences that can be assembled to create complex biological systems.