Our Manufacturing Capabilities and Capacity
Excellence in Manufacturing
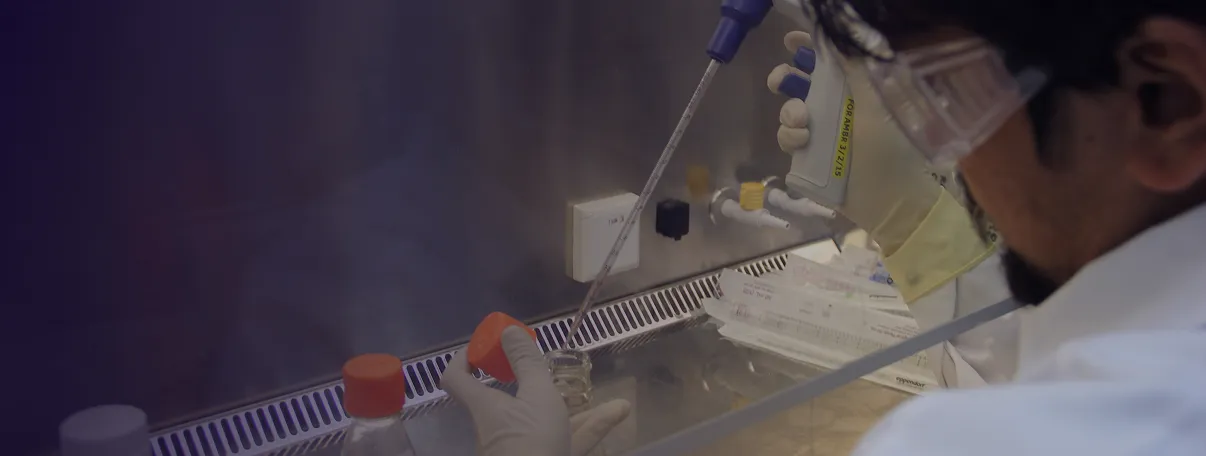
Dr. Reddy's manufacturing facilities are equipped with mammalian and bacterial cell culture units, including advanced stainless steel bioreactors and India’s first single-use biotechnology platform, reducing cross-contamination risks. The facilities include cutting-edge fill finish capabilities for vials and pre-filled syringes (PFS), supported by high-precision filling lines, visual inspection systems, and fully automated packaging. With a capacity to produce 12 million vials and 18 million PFS annually, backed by a $200 million investment and 350+ technical staff, Dr. Reddy’s meets global quality standards with accreditations from regulatory bodies across the Europe, Latin America and multiple international markets.



Built for Capacity
Dr. Reddy's Biologics combines scale, flexibility, and advanced technologies to meet diverse Biologics manufacturing needs. What’s on the table? As a Biosimilars Contract Manufacturing Organisation, we are well equipped with world-class manufacturing capabilities. A quick look below.
Quick Figures
We Meet Global Standards

- News
- 29-07-2024
Dr. Reddy’s biosimilar gets recommendation from European Medicines Agency
- News
- 29-07-2024
Dr Reddy’s gets positive opinion from EMA for its rituximab biosimilar
- Publication
- 22-06-2016
Efficacy and safety of an anti-CD20 monoclonal antibody (Reditux) for the treatment of patients with moderate to severe rheumatoid arthritis following the failure of conventional synthetic disease-modifying anti-rheumatic drugs
- Publication
- 31-03-2017
Long‑term outcome of diffuse large B‑cell lymphoma: Impact of biosimilar rituximab and radiation

Sreedhari V
Product Dev.

Afziya
Reg. Affairs

Syed Idris
Quality

Deekshit Kumar
Product Dev.

Prashant
Warehouse
Bricks of Biologics, appreciates the contribution and celebrates our people who are the building blocks of Dr. Reddy's Biologics.
Bricks of biologics in general refers to bio-bricks as genetic building blocks which are DNA sequences that can be assembled to create complex biological systems.