Our Expertise and Offerings
In Fight Against Cancer
Our focus is on Chimeric Antigen Receptor CAR-T Cell therapy, an advanced treatment that harnesses the patient’s own immune system to fight cancer. These therapies offer new hope for patients with certain types of cancers that are resistant to conventional treatments.
At Dr. Reddy’s Biologics, we are developing two proprietary CAR-T products, designed to target the BCMA and CD19 antigens, which are associated with multiple myeloma and B-Cell malignancies, respectively.
About The Novel Technology
What are CAR-T Cells?
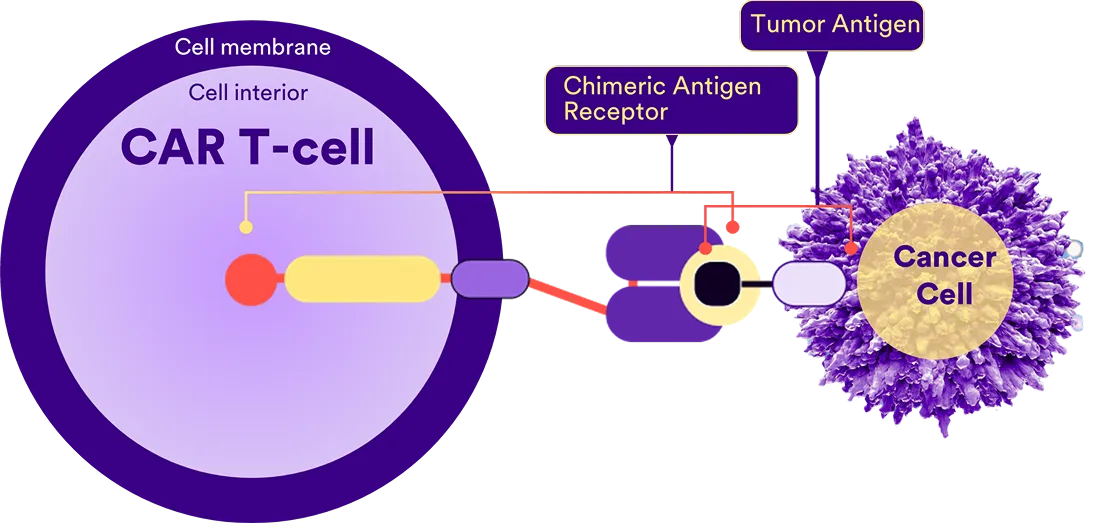
Chimeric Antigen Receptor T-Cells are genetically modified T-Cells.
What are T-Cells?
T-Cells are white blood cells essential to the immune system, protecting the body by identifying and destroying infected or cancerous cells.
CD8+ (Cytotoxic or Killer T-Cells) are Trained Assassins of the Immune System
These T-Cells can be modified to fight dangerous cancer cells. CAR-T Cell therapy is a type of immunotherapy that involves modifying a patient's T-Cells to recognise and attack cancer cells. This is done by adding special receptors to the T-Cells, enabling them to target specific proteins found on the surface of cancer cells.
How Are CAR-T Cells Made?
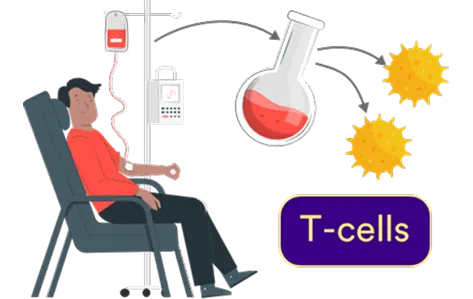
Collection
T-Cells are extracted from the patient’s blood.
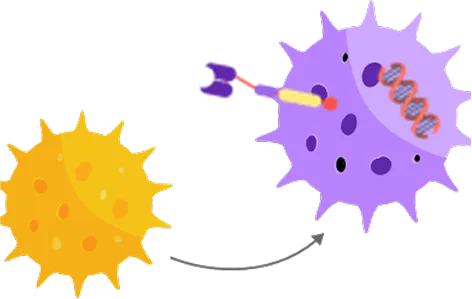
Modification
T-Cells are genetically engineered to have specialised receptors called CARs on their surface.
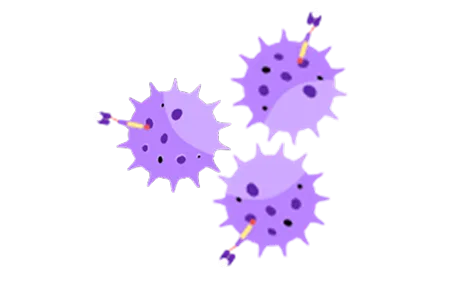
Multiplication
The modified T-Cells are cultivated in the lab to produce a large population of CAR-T Cells for effective cancer treatment.
Infusion
Reengineered T-Cells are infused back into the patient.
How CAR-T Therapy Works?
Immune Cell Activation
T-Cells release inflammatory cytokines, which are small proteins playing a crucial role in regulating immune responses and inflammation.
Cytotoxic Activity
T-Cells release cytotoxins, substances that can damage or destroy cells and tissues. These cytotoxins are critical for promoting cancer cell death by directly attacking and killing malignant cells.
Proliferation
T-Cells release interleukins, a group of cytokines crucial for cell signalling. These small proteins promote the growth, development, and division of immune cells, thereby strengthening the immune response.

Understand Vein-to-vein Process
Vein-to-vein process is a cycle of administering the treatment, from the collection of a patient's T-Cells to the infusion of the engineered CAR-T Cells back into the patient. It represents the full cycle of the therapy, ensuring that every step is completed in a timely and efficient manner for improved patient outcomes.
Our Manufacturing Excellence
Our State-of- the-art Facilities
We are overcoming the biggest challenges in CAR-T Cell production—scalability, cost, and accessibility. Our brownfield facility is equipped to overcome prevalent roadblocks.

Reduce bottlenecks in CAR-T therapy manufacturing.

Increase accessibility to cutting-edge cancer treatments.

Ensure faster turnaround times from cell collection to infusion.

Specialised CGT production units capable of scaling CAR-T therapies.
_0.svg)
Support labs and ancillary spaces for research, testing, and quality control.

Cryopreservation and storage areas for long-term cell storage.
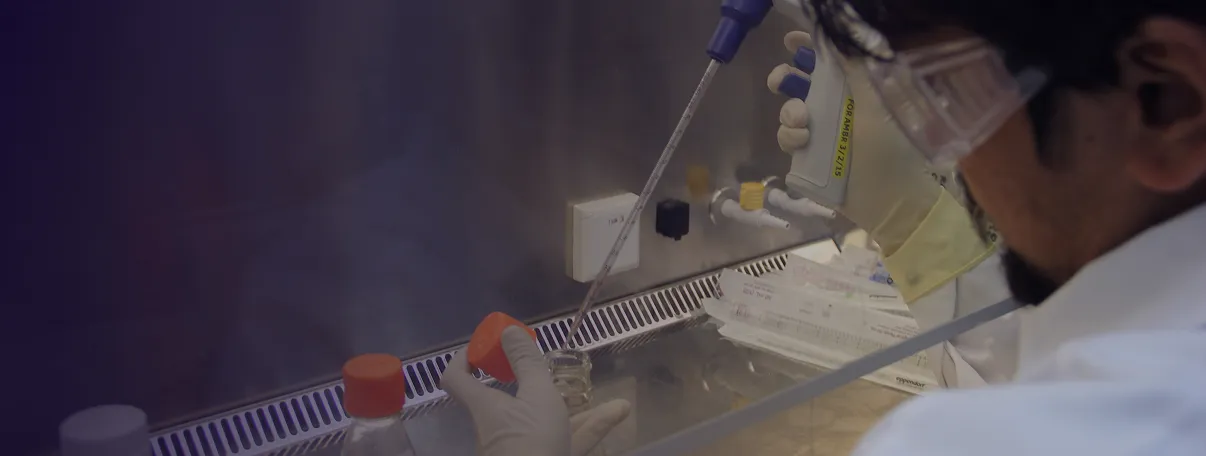
Collaboration is The Key
We are actively engaged in developing our clinical pipeline, with our initial focus on BCMA and CD19-targeting CAR-T therapies. We invite collaborations with hospitals, research institutions, and healthcare providers who share our passion for bringing the latest in cancer care to patients.
- Publication
- 06-08-2019
Comparison of the Efficacy of Innovator Rituximab and its Biosimilars in Diffuse Large B Cell Lymphoma Patients: A Retrospective Analysis
- Publication
- 30-09-2017
Infusion Related Hypersensitivity Reactions with Bio-similar Anti CD-20 Monoclonal Antibody Rituximab in Indian Patients: A Retrospective Study
- Article
- 30-09-2023
Temperature Upshifts in Mammalian Cell Culture: A Suitable Strategy for Biosimilar Monoclonal Antibodies?
- Article
- 16-07-2024
Mitigating target interference challenges in bridging immunogenicity assay to detect anti-t ocilizumab antibodies

Punitha P
Quality

Rajendra Siram
Manufacturing

Uday Kiran
Quality

Krishna Mohan B
Quality

Anirudha Ajay S
Manufacturing
Bricks of Biologics, appreciates the contribution and celebrates our people who are the building blocks of Dr. Reddy's Biologics.
Bricks of biologics in general refers to bio-bricks as genetic building blocks which are DNA sequences that can be assembled to create complex biological systems.