Key Figures
Our Products
Our labs, our scientists, and our research – we are all rooting for a healthier world. Each biosimilar product is a testament to our belief in accessibility for all. We are invested in product innovation in the industry and believe that the best biotech companies are ones that are always thinking ahead.
Our Journey
Explore Our Process of Excellence
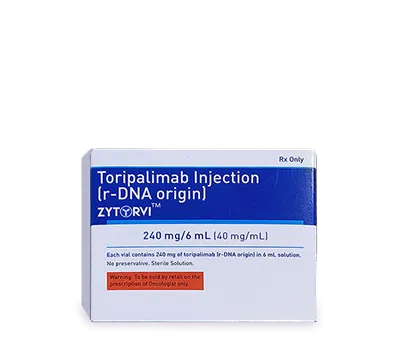
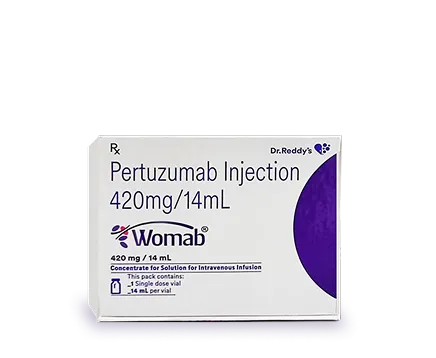
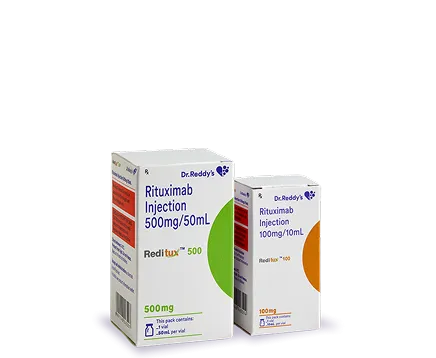
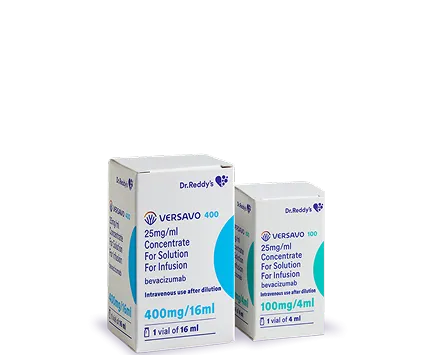
Dr. Reddy's Biologics is a global leader in the growing Biosimilars industry. With seven products already marketed in a large network of countries and an extensive pipeline under development, our priority remains quality, access, and affordability. We are proud of our end-to-end capabilities that ensure we can deliver what our patients need today.
- 1Product Development1
Product Development
Read More
- 2Manufacturing2
Manufacturing
Read More
- 3Clinical Development3
Clinical Development
Read More
- 4Quality4
Quality
Read More
- 5Logistics5
Logistics
Read More
1Product Development
Product Development is a fully integrated, end-to-end process designed for cost optimization and superior quality. These capabilities position us to deliver high-quality Biosimilar products efficiently and effectively.
- Leveraging Quality by Design (QbD) principles, our robust process design ensures consistent quality from the lab to manufacturing scale.
- Innovative formulation development, supported by digital tools, accelerates time-to-market.
- AI-driven analytics enable precise data insights and agile decision-making.
Cell-line development
Analytical development
Process development
Formulation development
Process scale-up
2Manufacturing
Dr.Reddy's Biologics manufacturing facilities are equipped with mammalian and bacterial cell culture units, including advanced stainless steel bioreactors and India’s first single-use biotechnology platform, reducing cross-contamination risks. The facilities include fill-finish capabilities for vials and pre-filled syringes (PFS), supported by high-precision filling lines, visual inspection systems, and fully automated packaging. Backed by a $200 million investment and 350+ technical staff, Dr.Reddy's Biologics meets global quality standards with accreditations from regulatory bodies across Europe, Latin America, South East Asia and multiple international markets.
Drug Substance Manufacturing (DS)
Drug Product Manufacturing (DP)
Packaging
Our accreditations and certificates
3Clinical Development
Dr. Reddy's Biologics clinical development capabilities cover the entire gamut of biologic products, from initial healthy volunteer to human factor, device bridging and patient-based studies to post-approval to post-marketing and real-world studies. The process ensures efficacy, safety, and immunogenicity , meeting global regulatory standards. With expertise in protocol development, regulatory submissions, and clinical trial execution, Dr. Reddy's team effectively manages complex trials. The company also excels in clinical trial analysis, offering data-driven insights that guide strategic decisions. Their medical writing and publication services ensure that all findings are accurately documented and shared with the scientific community.
Protocol development
Approvals for Clinical Trials
Clinical trial execution
Clinical trial analysis
Medical writing
Publication
4Quality
Dr. Reddy’s Regulatory and Quality Assurance teams uphold global compliance and the highest operational standards. With expertise in navigating complex international frameworks, they secure approvals from leading regulatory authorities. Critical Quality Attributes are rigorously monitored throughout manufacturing, ensuring consistency and continuous improvement. A centralised quality management system, supported by advanced tools like Laboratory Information Management System (LIMS) and Electronic Notebooks (ELN), guarantees data integrity and authenticity. This commitment ensures the delivery of safe, effective, and high-quality biologics to global markets.
5Logistics
A cornerstone of our business is the network, supply chain, and intuitive logistics we have built. Our commitment to access and reach is unwavering and our international distribution system and partners are a testament to that.
Storage
Supply chain - India
Supply chain - EU
Distribution

- Publication
- 12-02-2020
Pharmacokinetic Similarity and Comparative Pharmacodynamics, Safety, Efficacy, and Immunogenicity of DRL_RI Versus ReferenceRituximab in Biologics‑Naïve Patients with Moderate‑to‑SevereRheumatoid Arthritis: A Double‑Blind, Randomized, Three‑Arm Study
- Publication
- 06-12-2019
Randomized, Double-Blind, Pharmacokinetic Equivalence Trial Comparing DRL-Rituximab With MabThera in Patients With Diffuse Large B-Cell Lymphoma
- Publication
- 04-11-2022
Rituximab pharmacokinetic and pharmacokinetic–pharmacodynamics evaluation based on a study in diffuse large B-cell lymphoma: Influence of tumor size on pharmacokinetic and assessment of pharmacokinetic similarity
- Publication
- 31-12-2017
Comparative double-blind randomized trial of 2 rituximab products in patients with CD20+ diffuse large B-cell lymphoma (DLBCL), at Chicago, IL,

Milind Maske
Manufacturing

N K Bharath
Clinical Dev.

Amrutha
Quality

Jadav Darshan
IPM

Rohan Jain
Finance
Bricks of Biologics, appreciates the contribution and celebrates our people who are the building blocks of Dr. Reddy's Biologics.
Bricks of biologics in general refers to bio-bricks as genetic building blocks which are DNA sequences that can be assembled to create complex biological systems.